Welcome, fellow science enthusiasts and aspiring chemists! Get ready to dive deep into the wild and wonderful world of organic chemistry, where carbon atoms reign supreme and chemical reactions are as unpredictable as a fickle exothermic reaction. From the complexities of aromatic compounds to the beauty of stereochemistry, we will take you on a journey through the twisted, tangled web of covalent bonds and molecular structures. So buckle up, grab your lab coat, and prepare to unlock the mysteries of the molecular universe in all its organic glory!
Understanding the Basics of Organic Chemistry
So, you’ve decided to dive into the world of organic chemistry - the study of carbon compounds and their reactions. Congratulations! You’re in for a wild ride filled with molecules, reactions, and plenty of headaches.
First things first, let’s talk about the building blocks of organic chemistry – carbon atoms. These little guys are the foundation of all organic compounds and are known for their ability to form strong bonds with other elements. In fact, carbon can form up to four bonds with other atoms, making it incredibly versatile.
Now, let’s move on to functional groups. These are specific groups of atoms that give organic compounds their unique properties and reactivity. Some common functional groups you’ll encounter include alcohols, aldehydes, and carboxylic acids. Think of them as the spice that adds flavor to the bland world of carbon compounds.
When it comes to reactions in organic chemistry, there are a few key concepts to keep in mind. One of the most important is electron movement. Remember, electrons are the real MVPs in organic chemistry, driving reactions and determining the outcome. So, buckle up and get ready to solve puzzles with electrons as your guide!
The Importance of Carbon in Organic Compounds
Carbon is the ultimate rockstar of the organic chemistry world. Why, you ask? Well, let me break it down for you.
First of all, carbon is like the foundation of a killer party. It’s the life of the chemical reactions, bringing together all sorts of other elements to create some seriously cool compounds. Without carbon, organic chemistry would be as exciting as watching paint dry. Yawn.
Secondly, carbon is the king of versatility. It can form single bonds, double bonds, triple bonds – you name it, carbon can do it. It’s like the Swiss Army knife of the periodic table. Need a complex molecule? Call carbon.
Lastly, carbon is the driving force behind all living organisms. From the proteins in your muscles to the DNA in your cells, carbon is everywhere, making sure everything runs smoothly. It’s basically the MVP of the biological world. So next time you’re munching on a delicious apple or breathing in some fresh air, just remember – you’ve got carbon to thank for that!
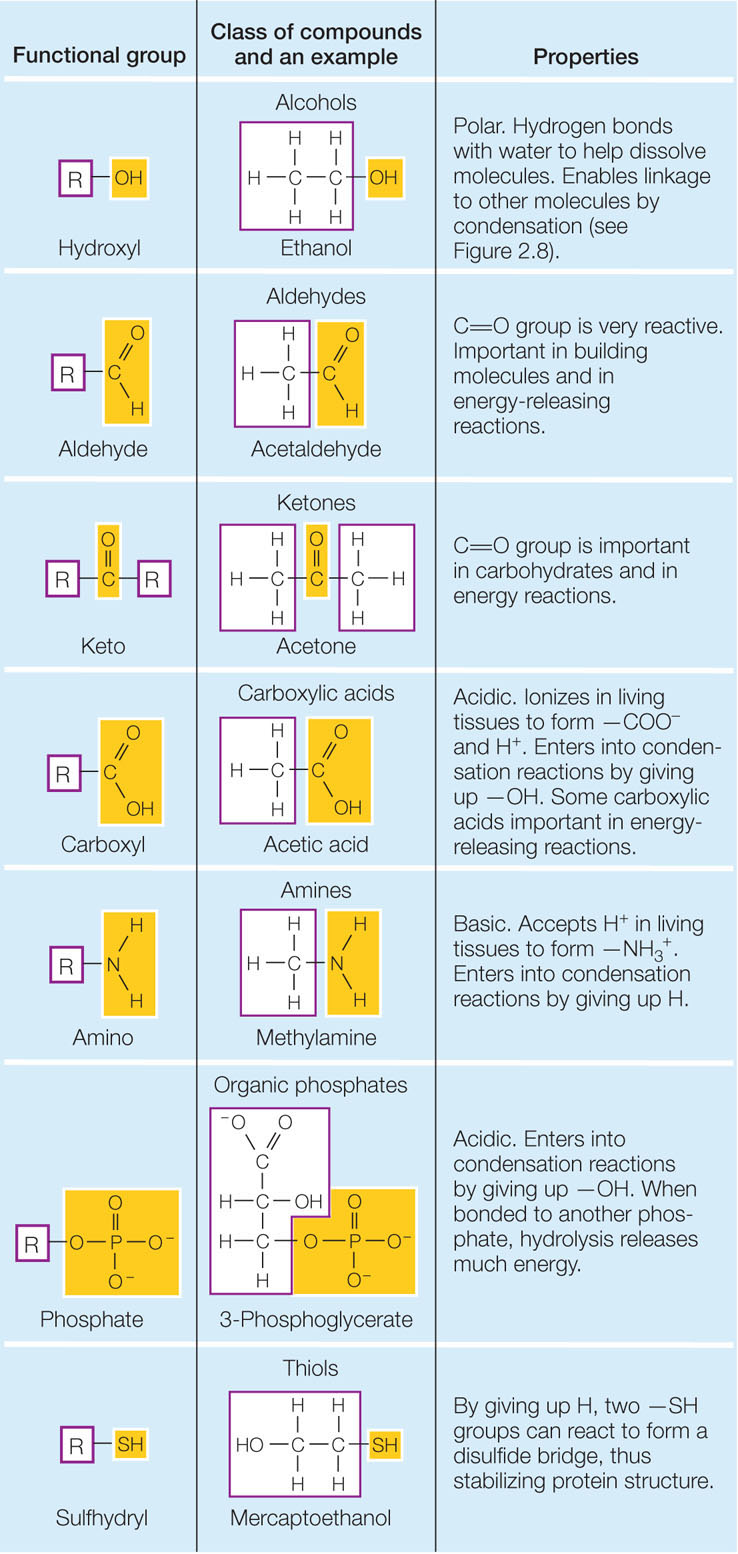
Exploring Functional Groups in Organic Molecules
Functional groups are like the Spice Girls of organic molecules – each one brings a unique flair to the party! From the sassy double bond in alkenes to the rebellious hydroxyl group in alcohols, there’s never a dull moment in the world of organic chemistry.
One of the coolest things about functional groups is how they can completely change the properties of a molecule. A simple alkane might be boring and uneventful, but add a dash of carboxyl group and suddenly you have a feisty carboxylic acid ready to cause some chemical chaos.
With so many different functional groups to choose from, it can be overwhelming to keep track of them all. But fear not, brave chemists! By studying the unique characteristics of each group, you’ll be able to predict how they’ll behave in different reactions and come out on top in the wild world of organic molecules.
So next time you’re feeling adventurous, grab your lab coat and safety goggles and dive into the fascinating world of functional groups. Who knows what kind of chemical shenanigans you’ll uncover along the way!
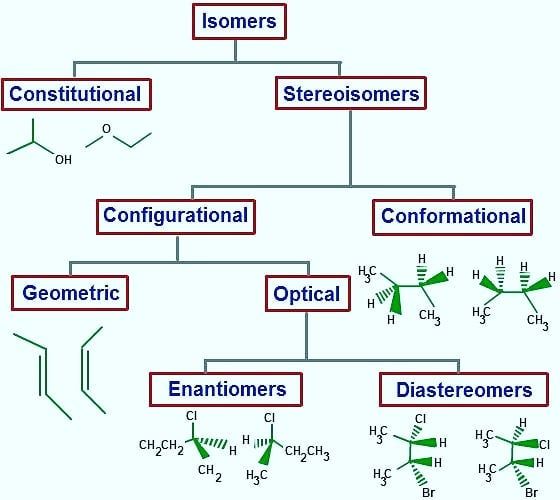
The Role of Isomerism in Organic Chemistry
Isomerism in organic chemistry is like having a twin brother who looks exactly like you but acts completely different. It’s that quirky phenomenon where molecules with the same molecular formula exist in multiple forms due to differences in their structural arrangements.
Imagine a game of molecular musical chairs where atoms switch seats to create new molecules with the same ingredients. These sneaky molecules trick us into thinking they’re all the same, but oh no, they have different properties and reactivities!
From the delightful dance of conformational isomers to the chaotic circus of stereoisomers, organic chemistry is full of surprises. It’s like a never-ending puzzle where we have to match shapes and colors to figure out which isomer is which. Sometimes they play nice and make our lives easier, but other times they’re like mischievous little gremlins causing havoc in our reactions.
So next time you’re faced with a sea of isomers, remember to keep your wits about you and embrace the madness of organic chemistry. It’s a wild ride full of twists and turns, but hey, that’s what makes it so darn fun!

Analyzing Reaction Mechanisms in Organic Synthesis
So, you’ve found yourself knee-deep in the world of organic synthesis, trying to decipher reaction mechanisms like you’re cracking a secret code. But fear not, dear chemist! With a little bit of patience and a dash of humor, you’ll soon be analyzing reaction mechanisms like a pro.
First things first, make sure you’ve got your trusty toolbox of organic chemistry knowledge at the ready. Brush up on your understanding of electron pushing arrows, intermediates, and transition states. And don’t forget to keep your trusty periodic table close by – you never know when you’ll need to consult it for some atomic insight.
Next, roll up your sleeves and dive headfirst into those curly arrows. Whether you’re breaking bonds, forming new ones, or just trying to make sense of it all, remember that practice makes perfect. And if at first you don’t succeed, just keep pushing those electrons around until you reach that a-ha moment!
And last but not least, don’t be afraid to think outside the box. Sometimes the most unexpected pathways lead to the most exciting discoveries in organic synthesis. So grab your thinking cap, channel your inner mad scientist, and let’s get analyzing those reaction mechanisms with gusto!
The Significance of Stereochemistry in Organic Compounds
Stereochemistry in organic compounds is like the spice of the chemical world – it adds flavor, complexity, and depth to an otherwise bland dish. Just like how a pinch of salt can make or break a recipe, the arrangement of atoms in a molecule can drastically alter its properties and reactivity. Here are a few reasons why stereochemistry is so significant:
- **Chirality**: Imagine trying to fit a left-handed glove on your right hand - it just wouldn’t work! In chemistry, chirality refers to the arrangement of atoms in a molecule that makes it non-superimposable on its mirror image. This can lead to different biological activities, flavors, and fragrances. So, remember kids, always check the label before using that hand lotion – you don’t want to end up smelling like a decomposing fish instead of a bouquet of roses!
- **Stereoselectivity**: Much like how picky eaters only like their food cooked a certain way, some reactions in organic chemistry have a preference for certain stereochemical outcomes. Whether it’s a syn or anti addition, or a preference for the R or S configuration, stereochemistry can make or break a reaction faster than you can say “stereocenter.”
So, the next time you’re feeling overwhelmed by the seemingly endless isomers and enantiomers in organic chemistry, just remember - stereochemistry is what gives life to the molecules around us. Embrace the chirality, revel in the stereoselectivity, and remember that without stereochemistry, the world of organic compounds would be as dull as a chemistry textbook without puns (spoiler alert: it’s pretty dull!).
FAQs
Why is organic chemistry so important?
Because without it, we wouldn’t have the building blocks for life! Plus, it helps us understand the chemical reactions that happen all around us every day.
What are some common examples of organic compounds?
Think about things like sugars, proteins, fats, and even the caffeine in your morning coffee. Organic chemistry is everywhere!
What makes organic chemistry so challenging?
Well, for starters, there are a ton of different molecules to memorize. And let’s not forget about all those tricky reactions to keep straight. It’s like trying to solve a never-ending puzzle!
How can someone succeed in studying organic chemistry?
Just like with anything else, practice makes perfect. Make sure to review your notes, do plenty of practice problems, and don’t be afraid to ask for help when you need it. Oh, and a little bit of luck never hurts either!
Can you give an example of a complex organic reaction?
How about the Grignard reaction? It involves adding a Grignard reagent to a carbonyl compound to form a new carbon-carbon bond. It’s a real brain teaser, but oh-so-satisfying when you finally figure it out!
—
So, What’s the Deal with Organic Chemistry?
Phew! We’ve taken a wild ride through the world of organic chemistry, and let me tell you, it’s been quite the adventure. From bonding with carbon to unraveling the mysteries of functional groups, we’ve covered it all.
But hey, don’t let all this talk of molecules and reactions intimidate you. At the end of the day, organic chemistry is just like a complicated puzzle waiting to be solved. So grab your lab coat, put on your safety goggles, and dive in. Who knows, you might just discover the next groundbreaking compound!
And remember, in the wacky world of organic chemistry, the only limit is your imagination. So go forth, my fellow chemists, and conquer the world of carbon compounds!



